Dennis M. Dixon Thomas J. Walsh
An antifungal agent is a drug that selectively eliminates fungal pathogens from a host with minimal toxicity to the host.
Amphotericin, nystatin, and pimaricin interact with sterols in the cell membrane (ergosterol in fungi, cholesterol in humans) to form channels through which small molecules leak from the inside of the fungal cell to the outside.
Fluconazole, itraconazole, and ketoconazole inhibit cytochrome P450-dependent enzymes (particularly C14-demethylase) involved in the biosynthesis of ergosterol, which is required for fungal cell membrane structure and function.
Allylamines (naftifine, terbinafine) inhibit ergosterol biosynthesis at the level of squalene epoxidase. The morpholine drug, amorolfine, inhibits the same pathway at a later step.
5-Fluorocytosine acts as an inhibitor of both DNA and RNA synthesis via the intracytoplasmic conversion of 5-fluorocytosine to 5-fluorouracil.

The development of antifungal agents has lagged behind that of antibacterial agents. This is a predictable consequence of the cellular structure of the organisms involved. Bacteria are prokaryotic and hence offer numerous structural and metabolic targets that differ from those of the human host. Fungi, in contrast, are eukaryotes, and consequently most agents toxic to fungi are also toxic to the host. Furthermore, because fungi generally grow slowly and often in multicellular forms, they are more difficult to quantify than bacteria. This difficulty complicates experiments designed to evaluate the in vitro or in vivo properties of a potential antifungal agent.
Despite these limitations, numerous advances have been made in developing new antifungal agents and in understanding the existing ones. This chapter summarizes the more common antifungal agents. Three groups of drugs are emphasized: the polyenes, the azoles, and one antimetabolite. Table 76-1 summarizes the most important antifungal agents and their most common uses.
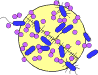
The polyene compounds are so named because of the alternating conjugated double bonds that constitute a part of their macrolide ring structure (Fig. 76-1). The polyene antibiotics are all products of Streptomyces species. These drugs interact with sterols in cell membranes (ergosterol in fungal cells; cholesterol in human cells) to form channels through the membrane, causing the cells to become leaky (Fig. 76-2). The polyene antifungal agents include nystatin, amphotericin B, and pimaricin.
FIGURE 76-1 Structures of some common antifungal agents.
FIGURE 76-2 Generalized fungal cell depicting the sites of action of the common antifungal agents.
Amphotericin B is the mainstay antifungal agent for treatment of life-threatening mycoses and for most other mycoses, with the possible exception of the dermatophytoses. Discovered by Gold in 1956, it can truly be said to represent a gold standard. Its broad spectrum of activity includes most of the medically important moulds and yeasts, including dimorphic pathogens such as Coccidioides immitis, Histoplasma capsulatum, Blastomyces dermatitidis, and Paracoccidioides brasiliensis. It is the drug of choice in treating most opportunistic mycoses caused by fungi such as Candida species, Cryptococcus neoformans, Aspergillus species, and the Zygomycetes. Resistance to this agent is rare, but is noteworthy for Pseudallescheria boydii, Fusarium spp., Trichosporon spp., certain isolates of Candida lusitaniae and Candida guilliermondii.
The drug must be administered intravenously and is associated with numerous side effects, ranging from phlebitis at the infusion site and chills to renal toxicity, which may be severe. A major advance in the use of this agent has resulted from an understanding of the mechanism of its renal toxicity, which is presumed to involve tubuloglomerular feedback. The suppression of glomerular filtration can be reduced by administering sodium chloride.
Nystatin was the first successful antifungal antibiotic to be developed, and it is still in general use. It is representative of the polyene antifungal agents developed later. The promise of its broad-spectrum antifungal activity is offset by host toxicity. Therefore, it is limited to topical use, where it has activity against yeasts such as the Candida species.
Pimaricin (natamycin), another polyene, is used topically to treat superficial mycotic infections of the eye. It is active against both yeasts and moulds.
The azole antifungal agents have five-membered organic rings that contain either two or three nitrogen molecules (the imidazoles and the triazoles respectively). The clinically useful imidazoles are clotrimazole, miconazole, and ketoconazole. Two important triazoles are itraconazole and fluconazole. In general, the azole antifungal agents are thought to inhibit cytochrome P450-dependent enzymes involved in the biosynthesis of cell membrane sterols.
Ketoconazole set the stage for the orally administered antifungal azoles. It can be administered both orally and topically and has a range of activity including infections due to H capsulatum and B dermatitidis, for which it is often used in nonimmunocompromised patients. It is also active against mucosal candidiasis and a variety of cutaneous mycoses, including dermatophyte infections, pityriasis versicolor, and cutaneous candidiasis. It is not indicated for treatment of aspergillosis or of systemic infections caused by yeasts.
The triazoles (fluconazole, itraconazole) have become the standard for the azoles, and have replaced amphotericin B for managing certain forms of the systemic mycoses. Fluconazole is now routinely used to treat candidemia in non-neutropenic hosts, and is gaining acceptance for use in cryptococcosis and selected forms of coccidioidomycosis. Itraconazole has proven to be effective for histoplasmosis, blastomycosis, sporotrichosis, coccidioidomycosis, consolidation treatment for cryptococcosis, and certain forms of aspergillosis. Fluconazole can be administered either orally, or intravenously. The licensed formulation for itraconazole is oral, but an intravenous formulation is under study, and could be a significant addition directed at bioavailability problems relating to absorption of the oral formulation.
Side effects are not as common with the azoles as with amphotericin B, but life-threatening liver toxicity can arise with long-term use. Liver toxicity noted with ketoconazole has been less problematic with the triazoles. Other side effects include nausea and vomiting. Drug interactions are a potential problem between azoles and other drug classes and include cyclosporin, certain antihistamines, anticoagulants, and antiseizure, oral hypoglycemic and other medications that are metabolized via similar pathways in the liver.
In contrast to the situation with antibacterial agents, few antimetabolites are available for use against fungi. The best example is 5-fluorocytosine, a fluorinated analog of cytosine. It inhibits both DNA and RNA synthesis via intracytoplasmic conversion to 5-fluorouracil. The latter is converted to two active nucleotides: 5-fluorouridine triphosphate, which inhibits RNA processing, and 5-fluorodeoxyuridine monophosphate, which inhibits thymidylate synthetase and hence the formation of the deoxythymidine triphosphate needed for DNA synthesis. As with other antimetabolites, the emergence of drug resistance is a problem. Therefore, 5-fluorocytosine is seldom used alone. In combination with amphotericin B it remains the treatment of choice for cryptococcal meningitis and is effective against a number of other mycoses, including some caused by the dematiaceous fungi and perhaps even by C albicans.
Griseofulvin is an antifungal antibiotic produced by Penicillium griseofulvum. It is active in vitro against most dermatophytes and has been the drug of choice for chronic infections caused by these fungi (e.g., nail infections with Trichophyton rubrum) since it is orally administered and presumably incorporated into actively growing tissue. It is still used in such instances but is being challenged by some of the newer azole antifungal agents. The drug inhibits mitosis in fungi.
Potassium iodide given orally as a saturated suspension is uniquely used to treat cutaneous and lymphocutaneous sporotrichosis. This compound, interestingly, is not active against Sporothrix schenckii in vitro. It appears to act by enhancing the transepidermal elimination process in the infected host.
Two other classes of antifungal agents represent new additions to topical treatment of the dermatomycoses in Europe. The two allylamines (naftifine and terbinafine) inhibit ergosterol synthesis at the level of squalene epoxidase; one morpholene derivative (amorolfine) inhibits at a subsequent step in the ergosterol pathway.
In vitro susceptibility testing with the fungi is not yet standardized, and the results of in vitro tests do not always compare to the results obtained in vivo. Therefore, preliminary selection of an antifungal agent for clinical use is made primarily on the basis of the specific fungal pathogen involved. The spectrum of activity for the licensed antifungal agents is well defined through the results of preclinical and clinical testing with the most common fungal pathogens. This approach is useful in avoiding selection of antifungals for species of fungi that are known to have primary resistance to the agent, but less useful in selecting antifungals for species that are known to develop secondary (drug induced) resistance to a particular agent.
Antifungal drug resistance has become an increasing problem with the development of a larger compendium of antifungal agents. Drug resistance to the polyene antifungals is almost always primary resistance rather than secondary resistance. That is, the susceptibility profiles for the species are characteristic and inherent, and rarely change in response to exposure to the agent. For example, amphotericin B-resistant species such as Pseudallescheria boydii and Candida lusitaniae are well known, and do not appear to have originated from exposure to the antifungal. Despite decades of widespread clinical use of amphotericin B in Candida albicans infections, the development of secondary resistance has been exceedingly rare. In contrast, both primary and secondary resistance to 5-fluorocytosine are known to occur for strains of Candida species, serving as the basis for restricting use of this agent to combination therapy with other antifungal drugs.
The question of fungal resistance to the azole drugs is considerably more complex and is currently under evaluation. Examples of both primary and secondary resistance are known for the medically important yeasts and selected azole antifungals. Candida krusei as a species is typically resistant to fluconazole. Candida albicans strains are typically susceptible to fluconazole and certain other azole antifungals, but there are increasing reports of resistance, especially in HIV-infected hosts having undergone repeated courses of azole antifungal therapy. The question of drug resistance is complicated by the limitations in the available susceptibility testing methodology and the ability to distinguish between microbiological and clinical drug resistance. The latter typically occurs when an inhibitory antifungal agent reaches the limits of its activity in a host with a decreasingly efficient immune system.
With the advent of the polyenes, azoles, and fluorocytosine, previously fatal infections can now be treated. However, as modern medicine continues to extend life through aggressive therapy of other life-threatening diseases such as cancer, there is an increasing population at risk for opportunistic fungal infections. Such patients represent a special challenge because they often are left with little host immune function. Therefore, chemotherapeutic agents should be fungicidal and not just fungistatic. The search continues for fungicidal agents that are nontoxic to the host. Research is also directed toward immunomodulating agents that can reverse the defects of native host immunity.
Casadevall A, and Scharff MD: Return to the past: The case for antibody-based therapies in infectious diseases. Clin Infect Dis 21:150-61, 1995
Como JA, and Dismukes WE: Oral azole drugs as systemic antifungal therapy. New Engl J Med 330:263-272, 1994
Dixon DM: In vivo models: evaluating antifungal agents. Methods Find Exp Clin Pharmacol 9:729, 1987
Espinel-Ingroff A, Shadomy S: In vitro and in vivo evaluation of antifungal agents. Eur J Clin Microbiol 8:352, 1989
Francis P, and Walsh TJ: Evolving role of flucytosine in immunocompromised patients: New insights into safety, pharmacokinetics, and antifungal therapy. Clin Infect Dis 15:1003- 1018, 1992
Fromtling RA (ed): Recent Trends in the Discovery, Development and Evaluation of Antifungal Agents. Prous, Barcelona, 1987
Galgiani JN: Antifungal susceptibility tests. Antimicrob Agents Chemother 31:1867, 1987
Graybill JR: New antifungal agents. Eur J. Clin Microbiol 8:402, 1989
Heidemann JF, Gerkens JF, Spickard WA: Amphotericin B nephrotoxicity in humans decreased by salt repletion. Am J. Med 75:476, 1983
Iwata K: Drug resistance in human pathogenic fungi. Eur J Epidemiol 8:407-421, 1992 Rinaldi MG, Dixon DM (eds): The evolving etiologies of invasive mycoses. Infect Dis Clin Practice 1994:3(suppl):S47-S112
Vanden Bossche H.: Molecular mechanisms of drug resistance in fungi. Trends in Microbiology 2:393-400, 1994
Walsh TJ: Recent advances in the treatment of fungal infections. Meth Find Exp Clin Pharmacol 9:769, 1987